Promilless products
Reliable alcohol testing is the cornerstone of responsible alcohol consumption. Promilless products provide an affordable, fast and reliable way to detect the blood alcohol level at any time. We offer you three saliva strip tests with the following limit variants: Zero, 0.2 and 0.5.

Promilless 0.0‰
When absolute zero tolerance is required.

Promilless 0.2‰
A test that measures BAC of 0.02% per mille.

Promilless 0.5‰
The maximum limit in our test range is 0.5 ‰.
How the saliva test strip works
The test can be interpreted within two minutes. Do not eat, drink or smoke 15 minutes before using the test – chewing gum included. Prior to the test, ensure you have saliva in your mouth.
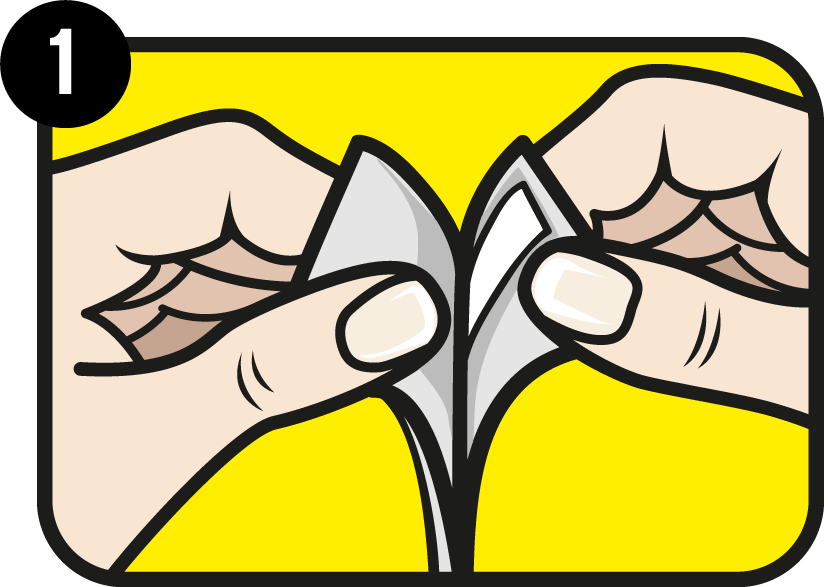
Get started
Remove the test strip from the foil packaging.

Dip in saliva
Dip the test strip in saliva for 2 seconds so that the green-yellow test area is against your tongue. Even the slightest darkening of the yellow test area means there is alcohol in the blood.
The green control area has darkened
Only the green control area has darkened: Your blood alcohol content is less than the limit indicated in the test. In Zero product: your blood alcohol level is zero per mill.
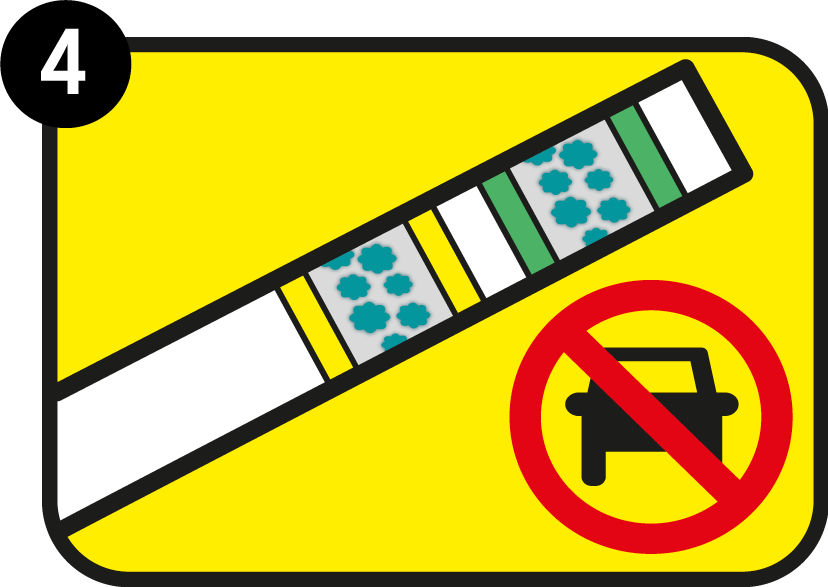
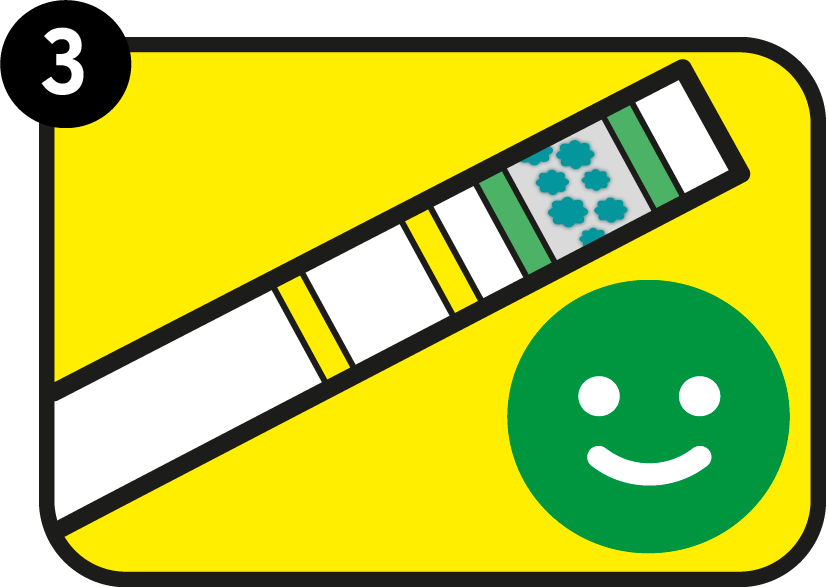
Also the yellow area has darkened
Both the green control area and the yellow test area have darkened: your blood alcohol content exceeds the limit indicated in the test. In Zero product: your blood alcohol level is more than zero per mill.
Availability
Promilless products are available in all well-stocked grocery stores as well as in Motonet stores. Please note that the product range varies from retailer to retailer. In grocery stores, our products can most often be found in the Intimate Hygiene department. Kesko stores sell exclusively 0.5 ‰ packs that have two tests instead of one.
Contact us: sales@promilless.com
Product technology and research
-
How is biochemistry connected to the products?
Detection of alcohol in saliva and other analytes is based on widely known and used biochemical reactions. The developed biochemical inks are printed and dispensed on a base, where they react with saliva and form a color reaction that provides the user with clear and unambiguous information about the test result.
-
Result readability and reliability
The test strip has a self-testing feature, which indicates the functionality of the strip to the user, taking in to account any possible incorrect storage, the end of the product’s life cycle and incorrect use conditions.
The result of the test strip is clearly readable from the darkening of the green control area and the yellow test area. The result can be read as soon as the slightest color reaction has occurred. The color does not need to be separately compared with color scales.
-
Intended use
Promilless alcohol test is fast and easy-to-use quick test designed to be used for detecting alcohol content from human saliva. The test is suitable for assessing blood alcohol content and can be used by consumers, healthcare professionals and authorities
Promilless alcohol test isdesigned to be used by consumer for checking the BAC (blood alcohol content) level, either under or above 0.05% OR 0.02% AND in Promilless Zero BAC levels of either under or above 0.00%. The Promilless alcohol test is a CE-marked Class I medical device.
-
Research references
Several scientific studies have shown that the alcohol content in saliva and blood go hand-in-hand (McColl, et al. 1979, Jones 1979a, Jones 1979b, Haeckel & Bucklitsch 1987, Jones 1993 and Gubala & Zuba, 2003) *.
The measurement of blood alcohol content from saliva is scientifically proven to be a reliable method.
Source:
*Gubala, W. & Zuba, D. (2003) Gender differences in the pharmacokinetics of ethanol in saliva and blood after oral ingestion. Pol. J. Pharmacol. 55: 639-644.Haeckel, R. & Bucklitsch, I. (1987) The comparability of ethanol concentrations in peripheral blood and saliva. The phenomenon of variation in saliva to blood concentration ratios. J. Clin. Chem. Clin. Biochem. 25(4): 199-204.Jones, A.W. (1979a) Inter- and intra-individual variations in the saliva/blood alcohol ratio during ethanol metabolism in man. Clin. Chem. 25(8): 1394-1398.Jones, A.W. (1979b) Distribution of ethanol between saliva and blood in man. Clin. Exp. Pharmacol. Physiol. 6(1): 53-59.Jones, A.W. (1993) Pharmacokinetics of ethanol in saliva: comparison with blood and breath alcohol profiles, subjective feelings of intoxication, and diminished performance. Clin. Chem. 39(9): 1837-1844.McColl, K.E., Whiting, B., Moore, M.R. & Goldberg, A. (1979) Correlation of ethanol concentrations in blood and saliva. Clin. Sci. (Lond) 56(3): 283-286.
-
Conclusions from scientific studies
Instant test that use saliva as an analytic are reliable indicators in determining the blood alcohol content (Jones 1995, Degutis et al. 2004)*.
The functionality of the test is based on a generally known reaction (Honchar 1978, Prencipe 1987)**.
Source:
* Jones, A.W. (1995) Measuring ethanol in saliva with the QED Enzymatic test device: Comparison of results with blood- and breath-alcohol concentrations. J.Anal. Toxicol. 19: 169-174.* Degutis, L.C., Rabinovici, R., Sabbaj, A., Mascia, R. & D’Onofrio, G. (2004) The saliva test strip is an accurate method to determine blood alcohol concentration in trauma patients. Acad. Emerg. Med. 11(8): 885-887.
**Honchar, M.V. (1978) A sensitive method for quantitative analysis of hydrogen peroxide and oxidase substrates in biological samples. Ukr. Biokhim. Zh. 70(5): 157-163.** Prencipe, L., Iaccheri, E. & Manzati, C. (1987) Enzymatic ethanol assay: a new colorimetric method based on measurement of hydrogen peroxide. Clin. Chem. 33(4): 486-489.
-
Clinical study
Prospective, open label, single-centre study determining the efficiency, sensitivity, specificity, positive and negative predictive value of the saliva alcohol test in healthy adults compared to conventional blood alcohol and breathalyzer measurements
- In the research programme, the functioning of the Promilless test was tested for 50 research subjects and compared to the research subjects’ blood samples as well as the results of breathalysers intended for professional and authority use. There has been a total of 300 test events.
- The planning and implementation of the research protocol have been carried out by an independent party.
- The research has been approved by the Ethics Committee of Pohjois-Savo Hospital District.
- The research has been carried out in April-May 2016.
Promilless products have developed in cooperation with ![]() .
.

